- Research Project
LRBA as regulator of co-stimulation
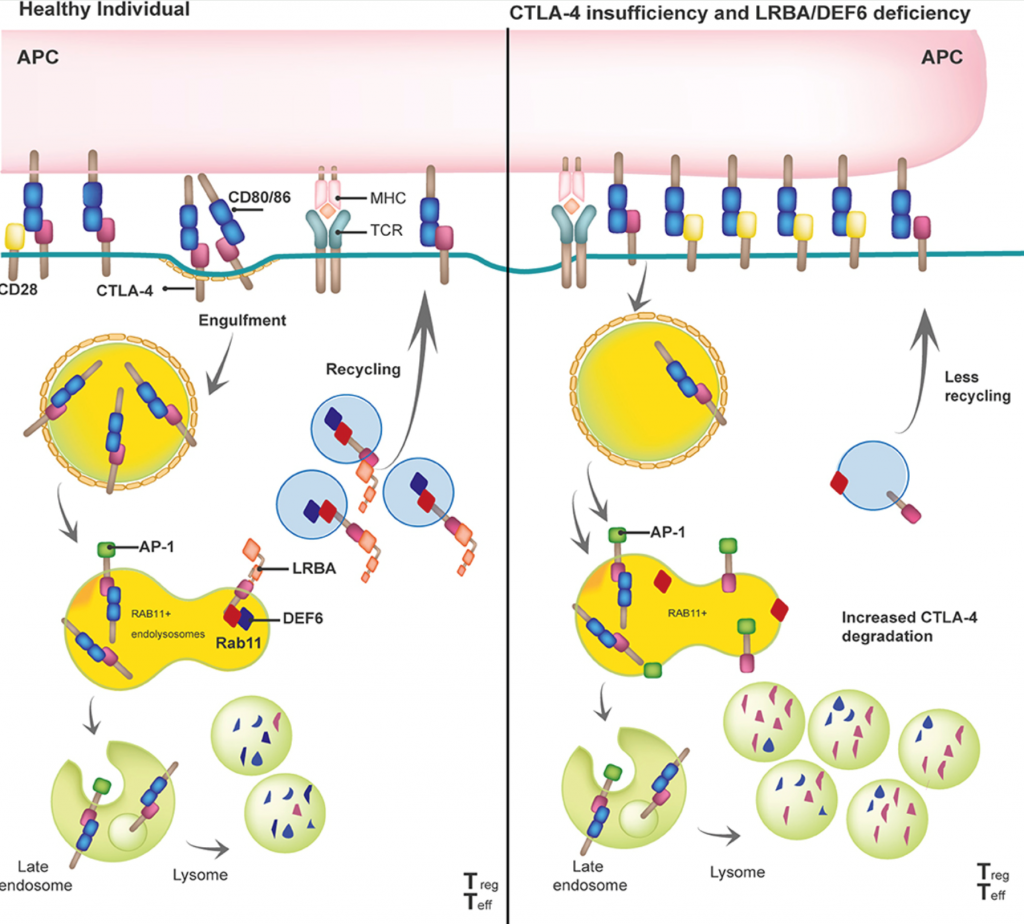
LRBA regulates the availability of CTLA4, autophagy, and cell metabolism
In 2012 we identified LRBA as being mutated in patients with autosomal recessive immune dysregulation and immunodeficiency (Lopez-Herrera et al., Am J Hum Genet, 2012). Following this discovery, we found that LRBA regulates autophagy. Others (Lo et al.; Science, 2015) identified LRBA as a key regulator of CTLA-4 trafficking. This project now evaluates the role of LRBA in cancer, its role in cell metabolism, and how we can use this knowledge for human diseases. This project is funded by the Wilhelm Sander-Stiftung, Förderantrags-Nr.2023.115.1.
References
- Gámez-Díaz L, Seidel MG. Different Apples, Same Tree: Visualizing Current Biological and Clinical Insights into CTLA-4 Insufficiency and LRBA and DEF6 Deficiencies. Front Pediatr. 2021;9:662645. doi: 10.3389/fped.2021.662645.
- Gámez-Díaz L, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. 2016;137(1):223-30. doi: 10.1016/j.jaci.2015.09.025.
- Lopez-Herrera G, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90(6):986-1001. doi: 10.1016/j.ajhg.2012.04.015.
Contact

Dr. Hannah van Dijk
E-Mail
Research Projects:
We're always here to help. Contact us today.

